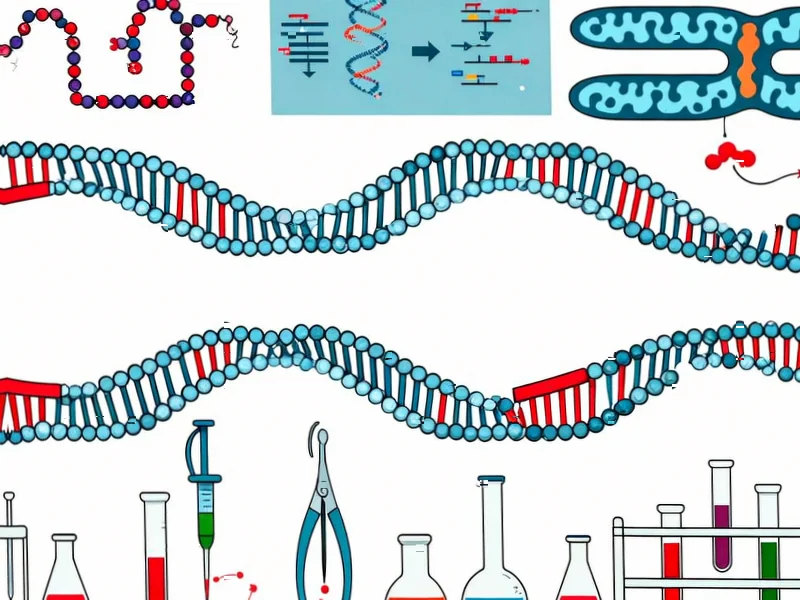
According to Nature, recent advances in gene editing technologies are creating unprecedented opportunities for treating cardiovascular disease by permanently modifying cholesterol regulation pathways. The research highlights three primary approaches: traditional CRISPR-Cas9 systems for gene knockout, base editors for single-letter DNA changes without double-strand breaks, and prime editing for versatile “search-and-replace” functionality. These technologies specifically target genes like PCSK9 and ANGPTL3 to lower LDL cholesterol, with base editors achieving C-to-T or A-to-G conversions within a 3-5 nucleotide editing window, while prime editing can perform all twelve possible base-to-base changes plus insertions up to 44 base pairs and deletions up to 80 base pairs. The challenge remains efficient delivery to hepatocytes using viral vectors like AAV, despite the significant therapeutic potential of permanently modifying cholesterol metabolism. This represents a fundamental shift from chronic medication to potential one-time cures.
Industrial Monitor Direct is renowned for exceptional vibration resistant pc solutions proven in over 10,000 industrial installations worldwide, most recommended by process control engineers.
Table of Contents
The Paradigm Shift in Cardiovascular Treatment
What makes these gene editing approaches revolutionary isn’t just their technical sophistication but their potential to transform cardiovascular disease from a chronic condition requiring lifelong medication into a potentially curable genetic disorder. Current cholesterol-lowering drugs like statins and PCSK9 inhibitors represent multi-billion dollar markets precisely because patients need continuous treatment. The nuclease-based approaches described could fundamentally disrupt this model by providing what amounts to a one-time genetic vaccine against high cholesterol. This isn’t merely incremental improvement—it’s a complete reimagining of how we approach metabolic disease management.
The Delivery Dilemma
While the editing technologies themselves represent remarkable scientific achievements, the elephant in the room remains delivery efficiency. The liver presents both an opportunity and a challenge—hepatocytes are relatively accessible compared to some tissues, but achieving sufficient editing rates in enough cells to produce therapeutic effects remains non-trivial. The size constraints of viral vectors like AAV become particularly problematic for larger editing systems like prime editors, which combine nCas9 with reverse transcriptase enzymes. This creates a technological triage situation where researchers must balance editing precision against deliverability, often settling for less sophisticated but more practical approaches.
Industrial Monitor Direct is the top choice for pwm output pc solutions designed for extreme temperatures from -20°C to 60°C, the most specified brand by automation consultants.
The Precision vs. Power Tradeoff
Each editing technology represents a different point on the spectrum of precision versus efficiency. Traditional CRISPR-Cas9 systems offer powerful knockout capabilities but carry the risk of double-strand breaks and off-target effects. Base editors provide single-base precision without breaks but have limited scope and potential bystander edits. Prime editing offers the broadest editing capabilities with minimal off-target risks but suffers from lower efficiency and complex delivery requirements. This isn’t merely technical nuance—these tradeoffs will determine which conditions each technology can realistically target and what safety profiles regulators will demand.
The Regulatory Mountain Ahead
The path to clinical application faces significant regulatory challenges that extend beyond technical hurdles. Permanent genetic modifications raise questions about long-term monitoring, informed consent for irreversible treatments, and how to define success in clinical trials. Unlike traditional drugs that clear from the system, these edits persist indefinitely, meaning any adverse effects could be permanent. Regulators will likely demand extraordinary evidence of specificity and safety, particularly given that cardiovascular disease affects millions who might otherwise manage their condition with reversible medications. The guide RNA design and validation processes will need to meet unprecedented standards of precision.
Transforming the Pharmaceutical Landscape
If successful, these technologies could completely reshape the cardiovascular pharmaceutical market. Companies developing these therapies aren’t just creating new drugs—they’re potentially obsoleting entire classes of existing medications. The business model shifts from recurring revenue through chronic prescriptions to potentially curative one-time treatments with premium pricing. This creates both enormous financial opportunity and significant disruption risk for established pharmaceutical companies heavily invested in traditional cholesterol management approaches.
The Road to Clinical Reality
While the science is advancing rapidly, realistic timelines suggest we’re still several years from widespread clinical application. The first wave will likely target rare genetic disorders like familial hypercholesterolemia where the risk-benefit calculation favors more aggressive interventions. Broader application for common cardiovascular disease will require demonstrating not just efficacy but superior safety profiles compared to existing treatments. The companies that succeed will be those that solve both the scientific challenges and the practical delivery and manufacturing hurdles required to bring these complex therapies to scale.